BioTherapeutics Presents BT-11: A New First-in-Class Crohn’s Disease Therapeutic at Digestive Disease Week
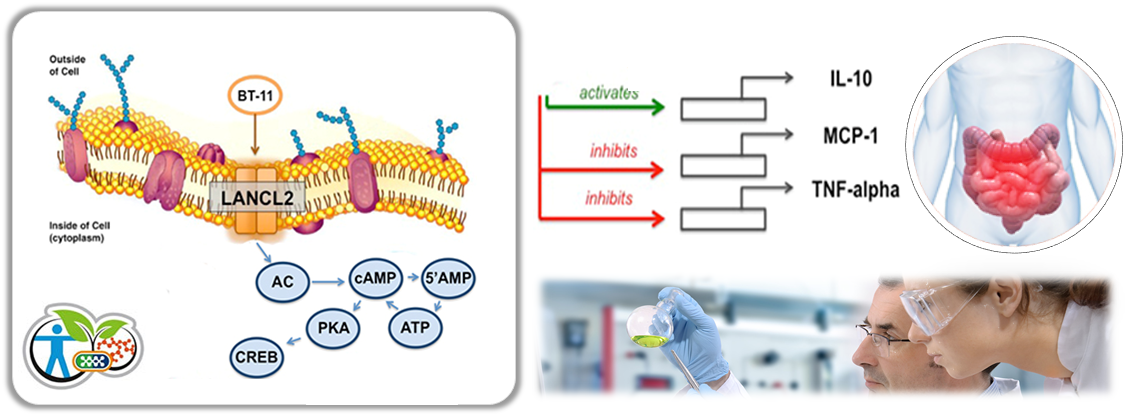
Inflammatory bowel disease (IBD) and its two main clinical manifestations—ulcerative colitis (UC) and Crohn’s disease (CD)—afflict over 1.4 million people in the U.S. and 4 million worldwide. Current therapies for IBD have limited efficacy and significant side effects such as infection, cancer, and death. Over 70% of CD patients undergo surgery even when being treated with current drugs, highlighting the important, unmet clinical need for safer and more effective therapeutics. Thus, discovering new first-in-class therapeutics for CD that regulate gastrointestinal (GI) inflammation more safely will completely transform the treatment paradigm and long-term management of this widespread and debilitating disease.
“During DDW we presented compelling preclinical and translational evidence demonstrating that BT-11 is a Novel Lanthionine Synthetase C-like 2 (LANCL2)-based first-in-class oral therapeutic for IBD,” said Dr. Josep Bassaganya-Riera, Director of NIMML and MIEP who presented at Digestive Disease Week® (DDW) during the annual meeting of the American Gastroenterological Association (AGA). “Further, our comparative therapeutic efficacy studies demonstrate that BT-11 is safer and more effective than current and IND drugs. Thus, BT-11 has the potential to be steroid- and biological-sparing and offers a safer and more effective alternative to current
medications.”
“LANCL2 is a novel therapeutic target for inflammatory and autoimmune diseases,” commented Dr. Raquel Hontecillas, the Co-Director of the NIMML. “Based on extensive preclinical studies, BT-11 is supported by strong animal pharmacology data in three validated mouse models of IBD, a benign safety profile, and human translational data in peripheral blood mononuclear cells (PBMC) from CD patients. BT-11 is a transformative orally active therapeutic that acts locally in the lower GI tract, and addresses a significant unmet clinical need for safer and more effective CD therapeutics.”
About DDW
Digestive Disease Week® (DDW) is the largest international gathering of physicians, researchers, and academics in the fields of gastroenterology, hepatology, endoscopy and gastrointestinal surgery. Jointly sponsored by the American Association for the Study of Liver Diseases (AASLD), the American Gastroenterological Association (AGA) Institute, the American Society for Gastrointestinal Endoscopy (ASGE) and the Society for Surgery of the Alimentary Tract (SSAT), DDW took place May 21-24, 2016 at the San Diego Convention Center, San Diego, CA. The meeting showcases more than 5,000 abstracts and hundreds of lectures on the latest advances in GI research, medicine and technology.
About BioTherapeutics Inc.
BTI Pharmaceuticals is a biotech company that develops disruptive first-in-class therapeutics for Crohn’s disease. BTI’s multifaceted approach synergistically combines the power of computational modeling with preclinical and clinical experimentation to accelerate the path to safer and more effective treatments for debilitating and widespread human diseases.
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
