GBCB Seminar “Modeling mucosal immune responses to Helicobacter pylori infection”, May 1, 2014
Modeling mucosal immune responses to Helicobacter pylori infection
Monica Viladomiu Pujol, GBCB Doctoral Candidate
Nutritional Immunology and Molecular Medicine Laboratory, Modeling Immunity to Enteric Pathogens
Advisor: Josep Bassaganya-Riera, Department of Biomedical Sciences and Pathobiology and Virginia Bioinformatics Institute
Co-advisor: Raquel Hontecillas, Virginia Bioinformatics Institute
Abstract
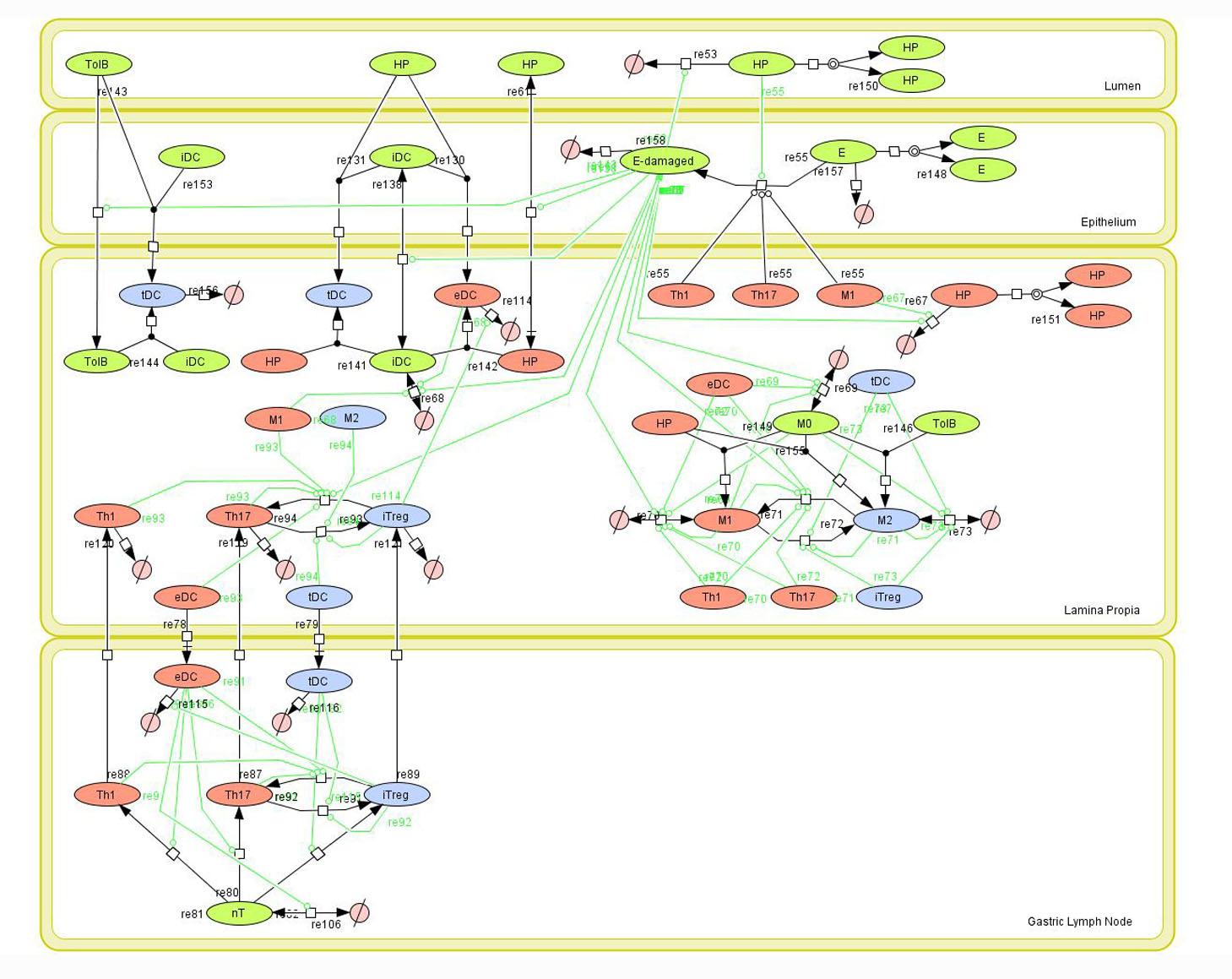
Helicobacter pylori is a gram negative, spiral-shaped bacterium with a unique capacity to colonize and chronically persist in 50% of the human population. H. pylori is the most common cause of gastric and duodenal ulcers, and about 15% of colonized individuals have a lifetime risk of developing gastritis, peptic ulcers or other gastric malignancies. Initially thought to be an exclusively extracellular pathogen , research on the immune response to its infection has mainly focused on the induction of CD4+ T cell responses [ 1 ] . However, recent data indicates that H. pylori can also replicate and persist within intracellular niches in gastric epithelial and immune cells, thus providing evidence for its role as a facultative intracellular bacterium. Based on our previous results showing that H. pylori infection induces Th1 and CD8+ T cells responses in pigs [ 2 , 3 ] , and preliminary mouse data pointing towards macrophages as critical regulators of colonization and gastric pathology, we are currently investigating the effector and regulatory mechanisms of host response induced by intracellular H. pylori infection. RNA-seq data from mouse macrophages co-cultured with H. pylori strain SS1 revealed two distinct phases of the host response to H. pylori ; an initial phase involving innate responses mediated by Toll-Like Receptors (i.e., TLR4, TLR2) and Nod-Like Receptorss (i.e., NLRC5, NLRP3 and NLRX1) and a second phase where the type 1 interferon response typical of viral and intracellular pathogen infections dominates. Notably, innate immune pathways intersect metabolic changes (i.e., cholesterol biosynthesis, mitochondrial oxidative phosphorylation, reactive oxygen species, lipid and amino acid metabolism). Together, these two phased innate responses and associated host metabolic changes are likely involved in the regulation of adaptive T cell responses at later stages of infection.
References
1. Carbo A, Bassaganya-Riera J, Pedragosa M, Viladomiu M , Marathe M, Eubank S, Wendelsdorf K, Bisset K, Hoops S, Deng X, Alam M, Kronsteiner B, Mei Y, Hontecillas R , Predictive computational modeling of the mucosal immune responses during Helicobacter pylori infection. PLoS One, 2013. 8 (9): p. e73365.
2. Kronsteiner B, Bassaganya-Riera J, Philipson C, Viladomiu M , Carbo A, Pedragosa M, Vento S, Hontecillas R. , Helicobacter pylori infection in a pig model is dominated by Th1 and cytotoxic CD8+ T cell responses. Infect Immun, 2013. 81 (10): p. 3803-13.
3. Kronsteiner B, Bassaganya-Riera J, Philipson N, Hontecillas R , Novel insights on the role of CD8+ T cells and cytotoxic responses during Helicobacter pylori infection. Gut Microbes, 2014. 5 (3).
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
