LANCL2: A Novel Therapeutic Target for Influenza
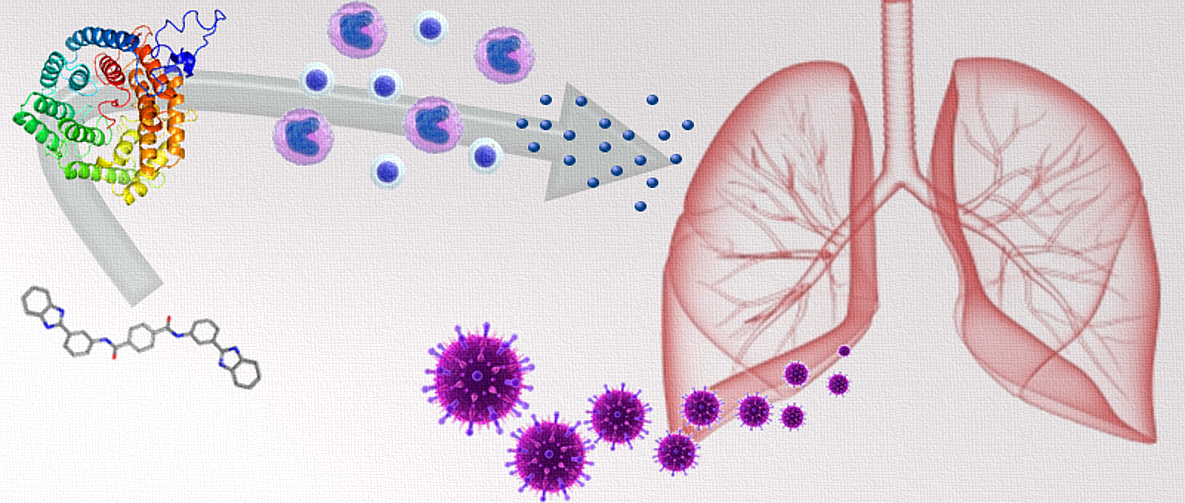
The Nutritional Immunology and Molecular Medicine Laboratory (NIMML) has recently explored the potential of activating lanthionine synthetase C-like 2 (LANCL2) to treat infectious diseases, particularly influenza virus infection. The work, which characterizes the effects of model LANCL2 ligand NSC61610 in influenza A infection of mice, is published within Frontiers in Immunology.
LANCL2 is an important molecule in the induction of regulatory responses that act to prevent excessive inflammation, among other functions. In line with this, initial experiments identified that mice lacking LANCL2 struggled to recover after the peak of influenza virus infection and experienced higher mortality. With the activation of LANCL2, NSC61610-treated groups had significantly lower disease severity and a 2-fold increase in survival rates.
“The combined observation that the genetic loss of LANCL2 and the pharmacological activation of LANCL2 through a small molecule both impact the overall disease response during influenza but in opposite patterns is strong validation that LANCL2 is an important target in the control of general immune responses and potentially specifically within responses to viral pathogens,” said Andrew Leber, NIMML Ph. D student in Genetics, Bioinformatics, and Computational Biology.
These beneficial effects of NSC61610 and LANCL2 were dependent on the production of IL-10 from macrophages and CD8+ T cells late in infection, which are able to drive resolution and repair responses within the lungs. The NIMML has also assessed the efficacy of abscisic acid (ABA), a proposed natural ligand of LANCL2, as a therapeutic for influenza infection in a 2013 publication in the Journal of Nutritional Biochemistry.
“While the immune effects of LANCL2 ligands are well understood in the gastrointestinal (GI) tract, less was known about what this receptor does in the respiratory tract. We’ve shown conclusively that not only does LANCL2 activation ameliorate disease activity and lung inflammatory pathology, it does it through a mechanism involving upregulation of lung IL-10 in influenza-infected mice,” said Raquel Hontecillas, co-Director of NIMML and the corresponding author of the paper.
While vaccines have been valuable against the spread of infections, they often require more than 6 months from the emergence of a particular strain to become fully developed. In contrast, the current option for those contracting disease, is anti-viral treatment such as Tamiflu. Unlike vaccines, numerous issues arise with anti-viral treatments, such as the need to early administration (before or immediately after first presentation of symptoms), the potential for emergence of resistant strains, and an inability to modulate host responses, which amount to only a 24-hour reduction in disease course on average.
When compared to Tamiflu, pharmacological activation of LANCL2 results in increased survival and accelerated recovery from influenza. Further, a common concern of immunoregulatory treatment is that it will result in a greater viral or bacterial load. However, the viral titer from the lungs of NSC61610-treated groups did not significantly differ from untreated groups.
“Anti-virals used to treat influenza such as Tamiflu target the virus rather than the immune response to the virus,” said Josep Bassaganya-Riera, the NIMML Director and a co-author of the paper. “We provide novel evidence that modulating the host response to influenza virus by activating LANCL2 can dramatically ameliorate survival, disease and pathology during influenza and possibly outperform Tamiflu, the current standard of care for treating infections with influenza virus.”
Beyond influenza infection, the role of LANCL2 has previously been characterized in gastrointestinal and metabolic disease, showing potent capabilities in a variety of diseases and validating efforts to advance the LANCL2 as a therapeutic target for infectious and immune-mediated diseases.
For More Information:
LANCL2 in response to H. pylori
LANCL2 in inflammatory bowel disease
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
