Researchers Discover Novel Immune Modulatory Properties and Clinical Efficacy of Conjugated Linoleic Acid in Crohn’s Disease Patients
BLACKSBURG, Va., March 16th, 2012 – A team of researchers at the Nutritional Immunology and Molecular Medicine Laboratory (NIMML) at the Virginia Tech, in collaboration with the Division of Gastroenterology and Hepathology at University of North Carolina School of Medicine and the Wake Forest Medical Center have reported new findings on the immune modulatory properties of conjugated linoleic acid (CLA) in Crohn’s disease (CD) patients. The two main clinical manifestations of Inflammatory Bowel Disease (IBD): CD and ulcerative colitis (UC), afflict over 1.4 million people in the United States with a worldwide prevalence reaching up to 396/100,000 persons. In addition, the risk of developing colorectal cancer increases by 0.5–1.0% yearly in IBD patients. These novel findings reported in the most recent edition of Clinical Nutritiona, were awarded the American College of Gastroenterology Presidential Poster of distinction for human clinical trial and help move researchers one step further in their efforts to develop safer and more efficacious interventions against IBD.
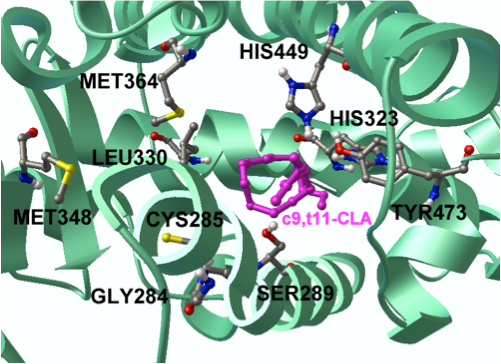
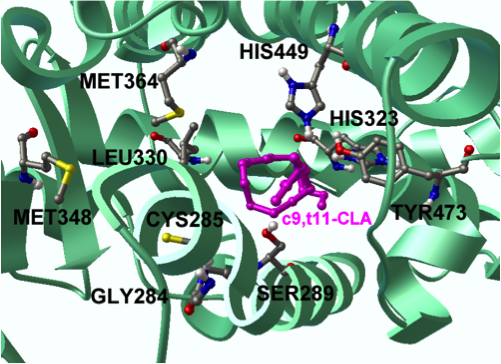 “CLA has been extensively investigated by NIMML since the Laboratory’s foundation in 2002. Our group published seminal papers reporting the discovery of novel mechanisms underlying the protective effects of CLA in colitis models. The new clinical findings in humans validate the predictions of those modeling efforts by demonstrating clinical efficacy and immune modulatory capacity of CLA. Specifically, we provide evidence that oral CLA-supplementation (6 g/d) for 90 days decreased production of inflammatory cytokines by peripheral blood CD4+ and CD8+ T cells, and improved clinical activity and quality of life in CD patients,” said Dr. Josep Bassaganya-Riera, a Professor of Immunology, principal investigator of this human clinical trial, and the Director of the NIMML and the Center for Modeling Immunity to Enteric Pathogens (MIEP).
“CLA has been extensively investigated by NIMML since the Laboratory’s foundation in 2002. Our group published seminal papers reporting the discovery of novel mechanisms underlying the protective effects of CLA in colitis models. The new clinical findings in humans validate the predictions of those modeling efforts by demonstrating clinical efficacy and immune modulatory capacity of CLA. Specifically, we provide evidence that oral CLA-supplementation (6 g/d) for 90 days decreased production of inflammatory cytokines by peripheral blood CD4+ and CD8+ T cells, and improved clinical activity and quality of life in CD patients,” said Dr. Josep Bassaganya-Riera, a Professor of Immunology, principal investigator of this human clinical trial, and the Director of the NIMML and the Center for Modeling Immunity to Enteric Pathogens (MIEP).
“In our recent open label study of CLA as a supplement in study subjects with mild to moderate CD there was a marked improvement in disease activity and quality of life in 50% of the subjects. CLA was well tolerated by all of the study subjects. These findings are very encouraging and will need to be verified in a randomized controlled trial,” said Professor Kim L. Isaacs, a Professor of Gastroenterology at the University of North Carolina at Chapel Hill.
“In terms of mechanism of action, we demonstrated that the preventive actions of CLA against colitis b and inflammation driven colorectal cancerc were abrogated in mice lacking peroxisome proliferator-activated receptor (PPAR) g in immune and epithelial cells. Indeed, mesalazine, a drug used for IBD treatment is known to exert potent PPAR g-activating effects. CLA holds an enormous potential as prophylactic for mild to moderately active IBD due to its limited site effects when compared to highly efficacious but unsafe synthethic PPAR g agonists such as rosiglitazone. Furthermore, we have demonstrated that probiotic bacteria can produce CLA locally and suppress colitis by targeting macrophage PPAR gd. Therefore, CLA can be administered directly in capsules or indirectly through CLA-producing probiotic bacteria,” said Dr. Raquel Hontecillas, an Assistant Professor of Immunology at NIMML.
“One of our goals in the NIMML is to develop safer and more effective therapies for human chronic inflammatory diseases from nature’s innovations. To achieve this goal, NIMML approaches span from computational modeling of mucosal immunitye to mechanistic and clinical experimentation. The validation of the anti-inflammatory actions of CLA in the gut is in line with our goal because CLA is a natural fatty acid found in milk and ruminant products. The fully integrated bioinformatics, nutrition and immunology experimentation capabilities of NIMML enable the acceleration of translational biomedical research from computational and mathematical modeling into the clinic. CLA is an example of an anti-inflammatory compound in a pipeline of naturally occurring and synthetic compounds (e.g., abscisic acid, eleostearic acid, terephthalanilides) with tremendous therapeutic and prophylactic potential as anti-inflammatories,” said Professor Josep Bassaganya-Riera.
For more information about the pre-clinical and clinical capabilities of the Laboratory please visit the NIMML Web Portal at www.nimml.org.
This clinical study was funded by a grant from Cognis GmbH, Monheim, Germany to Josep Bassaganya-Riera. Cognis is now part of BASF, The Chemical Company.
a Bassaganya-Riera, J., R. Hontecillas, W.T. Horne, M. Sandridge, H. Herfarth, R. Bloomfeld, and K. Isaacs (2012) Conjugated linoleic modulates immune responses in patients with Mild to Moderately active Crohn’s disease. Clinical Nutrition. In Press.
b Bassaganya-Riera, J., K. Reynolds, S. Martino-Catt, Y. Cui, L. Hennighausen, F. Gonzalez, J. Rohrer, A. Uribe Benninghoff, and R. Hontecillas (2004) Activation of peroxisome proliferator-activated receptor g and d by conjugated linoleic acid mediates protection from experimental inflammatory bowel disease. Gastroenterology. 127: 777-791.
c Evans, N.P., S.A. Misyak, E.M. Schmelz, R. Hontecillas and J. Bassaganya-Riera (2009) Conjugated Linoleic Acid Ameliorates Inflammation-Induced Colorectal Cancer in Mice through Activation of PPAR g. J. Nutr. 140(3): 515-21.
d Bassaganya-Riera, J., M. Viladomiu, M. Pedragosa, C. de Simone, A. Carbo, R. Shaykhutdinov, C. Jobin, J.C. Arthur, B. Corl, H. Vogel, M. Storr, and R. Hontecillas (2012) Probiotic bacteria produce conjugated linoleic acid locally in the gut that targets macrophage PPAR g to suppress colitis. PLOS One. In Press.
e Wendelsdorf, K., J. Bassaganya-Riera, R. Hontecillas, and S. Eubank (2010) Model of colonic inflammation: Immune modulatory mechanisms in inflammatory bowel disease. Journal of Theoretical Biology. 264(4):1225.
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
