Anti-inflammatory Mechanism of Host-Microbiota Cooperation Mediated by Helicobacter pylori
The Nutritional Immunology and Molecular Medicine Laboratory (NIMML) at the Biocomplexity Institute of Virginia Tech has discovered a new type of gastric immunoregulatory mononuclear phagocyte (MNP). In a new study published in the Journal of Immunology, they show, using a mouse model of infection, how these MNPs cooperate with the bacterium Helicobacter pylori to achieve high levels of colonization.
H. pylori is the main leading cause of gastroduodenal ulcer and gastric cancer. Due to the emergence of antibiotic-resistant strains, the World Health Organization (WHO) has recently classified H. pylori in group 2 high priority bacteria for which new treatments are urgently needed. However, most people carrying this bacterium remain healthy. Moreover, because some epidemiological studies have shown an inverse correlation between the presence of H. pylori in the stomach and the incidence of chronic morbidities like obesity, esophageal and cardial pathologies, childhood asthma, and childhood allergies some believe that this bacterium exerts beneficial effects in carriers.
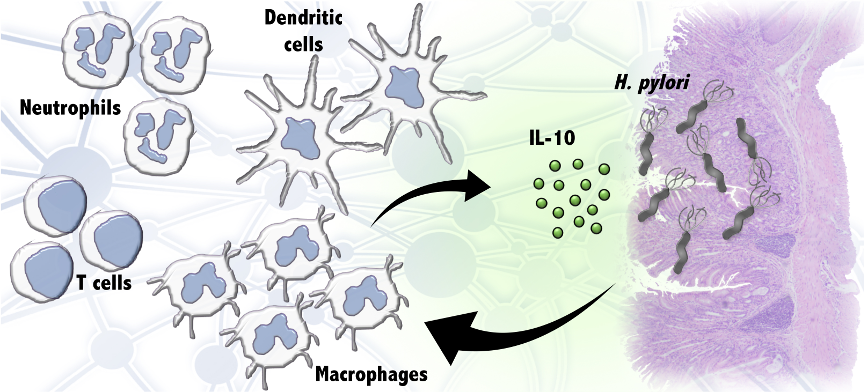
The study shows that this type of macrophage is present in the stomach of healthy uninfected mice in low numbers. However, 14 days after infection with H. pylori, these macrophages start accumulating in the gastric mucosa. To characterize their role during infection, they used chemically-induced macrophage depletion or a genetically modified mouse that lacks peroxisome proliferator-activated receptor g (PPARγ), a molecule that counteracts inflammatory signals. They found that both macrophage elimination and genetic ablation of PPARg resulted in lower levels of H. pylori colonization. They also found that the regulatory cytokine IL-10, which is produced in the stomach by this macrophage subset, is required for achieving high bacterial loads.
The improved mechanistic understanding of the complex and dynamic interactions between H. pylori and its human host is the first step in capturing human diversity and variation in response to the bacterium and predicting whether H. pylori infection will lead to a pathogenic or beneficial outcome.
“We have elucidated a novel mechanism by which regulatory myeloid cells create and anti-inflammatory environment in the stomach during H. pylori colonization that acts like anesthesia on the branch of the immune system that eliminates the bacteria. In this way, these macrophages cooperate with the infection allowing higher levels of colonization” said Raquel Hontecillas, Associate Professor of Immunology, NIMML co-Director, and corresponding author of the publication. “What our findings suggest is that having more H. pylori is not necessarily detrimental to the host. Moreover, there is the possibility that this mechanism of IL-10-induced host tolerance mediated by H. pylori at the GI mucosa might be implicated in providing the protection against asthma, allergies and immune-mediated diseases that has been associated with a carrier state”.
NIMML research iteratively incorporates advanced computational modeling to optimize pre-clinical, translational, and clinical experimentation. The integration of computational methods with experimental research focuses hypotheses and guides experimental research, saving time and laboratory costs.
“The computational and mathematical models, and data analytics platforms developed by NIMML have consistently predicted with >90% accuracy new mechanisms of immunoregulation and host-pathogen interaction, and response to treatment,” said Josep Bassaganya-Riera, Professor of Immunology and NIMML Director. “In fact, this publication validates one of our previous computational predictions and provides new evidence that CX3CR1+ MNP are central players in regulating the cooperative interactions between the host and H. pylori at the GI tract leading to anti-inflammatory responses. This mechanistic finding has profound implications for the development of precision medicine treatments at an individual, personalized level since most people that test positive for H. pylori are treated with antibiotics to eradicate the infection.”
The NIMML employs advanced computational tools in combination with experimental and clinical studies to develop safer, more effective precision medicine treatments for infectious and immune-mediated diseases. Previously, the NIMML reported the complex interaction between myeloid cells and H. pylori modeling LANCL2 and NLRX1 during the infection. The absence of such immunoregulatory molecules resulted in a decrease of bacterial loads in the gastric mucosa with similar characterization of an IL-10-dominated immune response. The newly reported mechanism of cooperation between H. pylori and the host response, confirms the important impact of microbiome-host interactions on human health, and delineates a possible path to new antimicrobial-free precision, personalized medicine treatments for GI infections.
For More Information:
Pig model of H. pylori infection
About NIMML
The NIMML Institute is a 501 (c) (3) non-profit public charity foundation focused on a transdisciplinary, team-science approach to precision medicine at the interface of immunology, inflammation, and metabolism. The NIMML Institute team has led numerous large-scale transdisciplinary projects and is dedicated to solving important societal problems by combining the expertise of immunologists, computational biologists, toxicologists, modelers, translational researchers, and molecular biologists. The Institute is headquartered in Blacksburg, VA. For more information, please visit www.nimml.org or contact pio@nimml.org.
